Thesis defence

Kamil Urbanek has got his PhD degree with distinction. Congratulations!
Photocatalytic CO2 reduction in the presence of composites of semiconductor photocatalysts
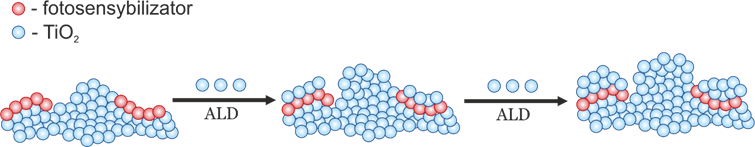
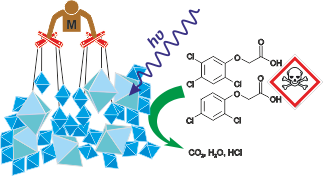
The urgent need to address the economic and environmental challenges posed by carbon dioxide has led to a concerted effort to develop techniques for its atmospheric transformation. Photocatalysis, leveraging solar energy, emerges as a promising avenue for mitigating CO2 levels and achieving a closed-loop carbon economy. However, traditional semiconductor photocatalysts like titanium dioxide demonstrate limited efficacy in CO2 reduction, especially when water serves as the electron donor. As water oxidation represents a less environmentally harmful process, this thesis explores TiO2 composites with other semiconductor photocatalysts to enhance photocatalytic activity. The investigation focuses on gas-phase reactors, enabling the utilization of air as a feed gas, thus potentially facilitating widespread adoption. A range of materials, including tin-iron, zinc-iron, cobalt, zinc-cobalt spinels, cadmium, copper and copper-cadmium sulfides, copper, tin, cerium, and tungsten oxides, and cobalt and copper tungstates, were characterized and tested for photocatalytic CO2 reduction, either alone or in conjunction with TiO2. While certain materials exhibited promising activity, others proved inert, even after attempts to activate them with platinum or palladium nanoparticles. Further experiments employed spectroelectrochemical techniques, photocurrent measurements, surface photovoltage measurements, and complementary oxidation processes to elucidate the mechanisms governing photocatalytic activity. Key inquiries included the potential efficiency enhancement of individual materials via composite formation, the viability of achieving S-scheme electron transfer with n-type and p-type semiconductor combinations, and the stability of multicomponent systems during photocatalytic CO2 reduction. Results demonstrated a notable improvement in photocatalytic activity for certain composites relative to their constituent materials, with mechanisms such as heterojunctions, S-Schemes, and TiO2 sensitization confirmed. Additionally, while combining p-type and n-type semiconductors did not always yield S-schemes or enhanced activity, the appropriate selection of materials with suitable Fermi levels proved crucial. Moreover, factors such as electrical contact between semiconductors and surface engineering influenced activity. Stability assessments revealed that some materials experienced decreased activity due to photocorrosion or active centre poisoning with CO2 reduction products. These findings underscore the complexity of optimizing photocatalytic CO2 reduction and highlight the importance of material selection and engineering for achieving efficient and stable systems.